This decision is much easier to make when you’re getting input from a trusted source. At CCCADA, we understand that the most important part of choosing a rehab center is finding the place that meets the individual’s every need. Call us today to speak directly with a trained addiction treatment specialist: (866) 781-3882.
On August 30 the Food and Drug Administration (FDA) introduced the agency’s Overdose Prevention Framework, meant to prevent drug overdoses and reduce deaths. A priority is expanding the availability of naloxone.
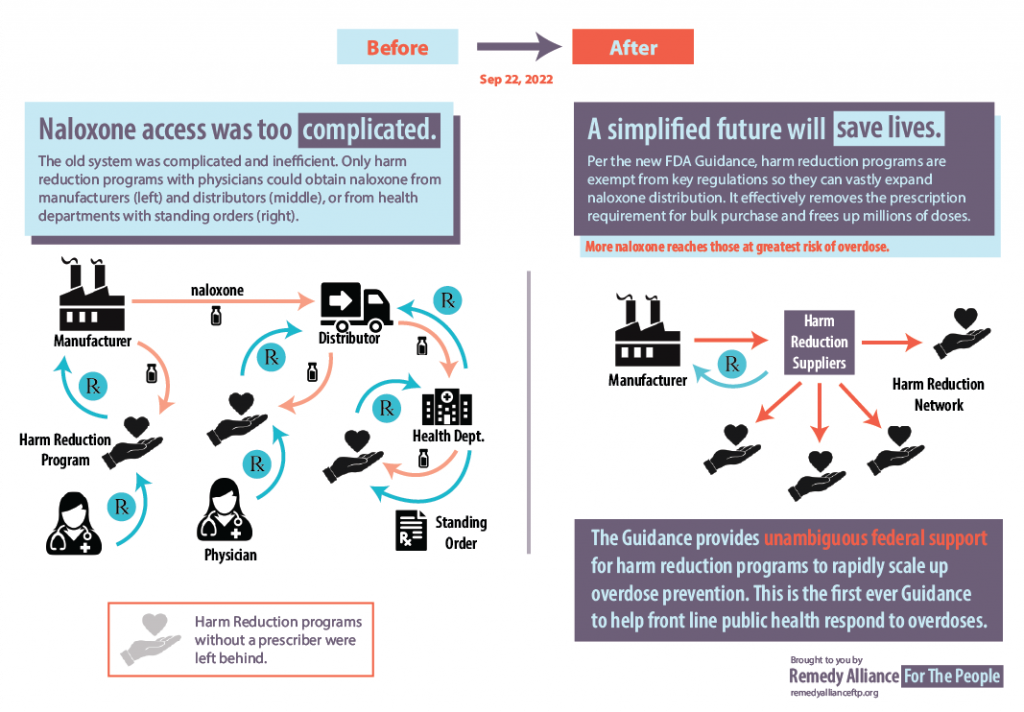
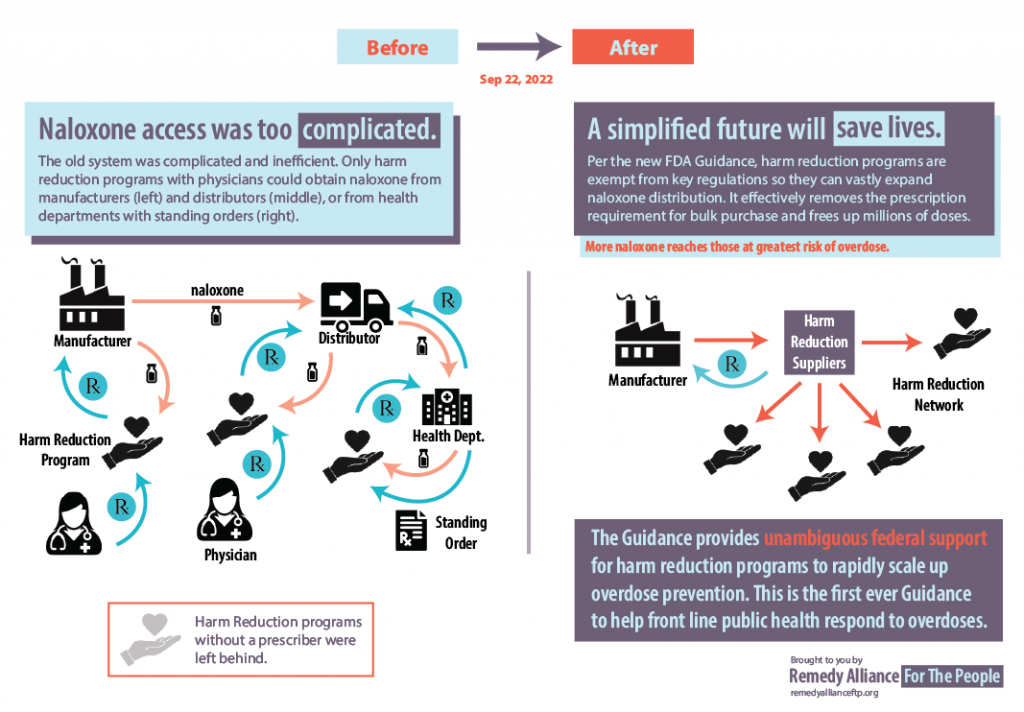
On Sept. 22, the FDA issued guidance which makes it easier for harm reduction organizations to purchase naloxone in bulk. The Exemption and Exclusion from Certain Requirements of the Drug Supply Chain Security Act (DSCSA) for the Distribution of FDA-Approved Naloxone Products During the Opioid Public Health Emergency took effect immediately.
The guidance clarifies that harm reduction organizations fall under the public health emergency exclusion and exemption from certain requirements for receiving naloxone products. The guidance includes a related compliance policy. “Entities such as harm reduction programs help save lives by making naloxone available in underserved communities,” the FDA noted in a blog the next day. “The agency intends to stand by these efforts by supporting harm reduction groups’ ability to acquire FDA-approved naloxone products.”
According to the FDA: “Every person who experiences an opioid overdose, whether it is with a prescribed medicine or an illicit drug, should have access to naloxone. With naloxone, anyone can help save the life of someone who is having an opioid overdose.”
The guidance will help address some of the obstacles to naloxone availability by aiding the ability of harm reduction organizations to obtain naloxone directly from manufacturers and distributors. The FDA noted that there is still more work to be done, because the current prescription-only status of naloxone is an additional barrier to availability.
Huge step
It’s a major step for harm reduction organizations, who do not normally applaud the federal government, but who were ecstatic about the announcement. “The FDA remains focused and committed to increasing naloxone access as we implement the Overdose Prevention Framework,” according to the agency’s blog. “We welcome working with harm reduction programs as well as all partners to reduce overdose deaths and, ultimately, achieve sustainable long-term recovery outcomes.”
Nabarun Dasgupta, Ph.D., co-founder and board chair of Remedy Alliance, the leader in harm reduction “buyers’ clubs” for naloxone, told AT Forum that Emergent Biosolutions made $434.2 million off of its Narcan nasal spray (which it bought from Adapt) in 2021, with a fourth quarter profit margin of 67%. “At around $50 per two-pack, the price is most definitely an issue,” said Dr. Dasgupta, an epidemiologist at the University of North Carolina at Chapel Hill. Naloxone is off-patent – a generic – which costs ”pennies to make,” he said. “So, while it’s not the intended goal of the FDA’s Guidance, it is a savvy move that enables free market forces to lower prescription drug prices, while simultaneously removing gatekeepers.”
Dr. Dasgupta said the FDA is actually making a “a deregulation move” which means that harm reduction programs can “now get access to cheaper (mostly injectable) naloxone by streamlining the procurement process, getting rid of barriers, and doing collective bargaining.”
Through the Remedy Alliance, vials are less than $4 each. So cost is obviously a massive barrier to scaling up for this life-saving drug.
For more on discount bulk naloxone, go to the Remedy Alliance at https://remedyallianceftp.org/
Comments welcome by FDA
In addition to making bulk-buying of naloxone available to harm reduction organizations, the FDA’s Overdose Prevention Framework includes:
Expanding availability and access to overdose reversal products, including naloxone, by supporting accelerated review of products and exploring over-the-counter accessSupporting development of novel overdose reversal productsSupporting development and authorization of fentanyl test strips to test human specimens at the point of care
For the guidance document, see https://www.fda.gov/media/161750/download.
The FDA is accepting comments on the guidance, although it is immediately in effect. For more information, please refer to https://www.regulations.gov and the docket number FDA-2022-D-1847.
The post FDA paves the way for harm reduction organizations to bulk-buy naloxone, thereby lowering the price appeared first on Addiction Treatment Forum.